

The cracking of hydrocarbons will be the main focus of our article today. This involves breaking a C-C single bond.
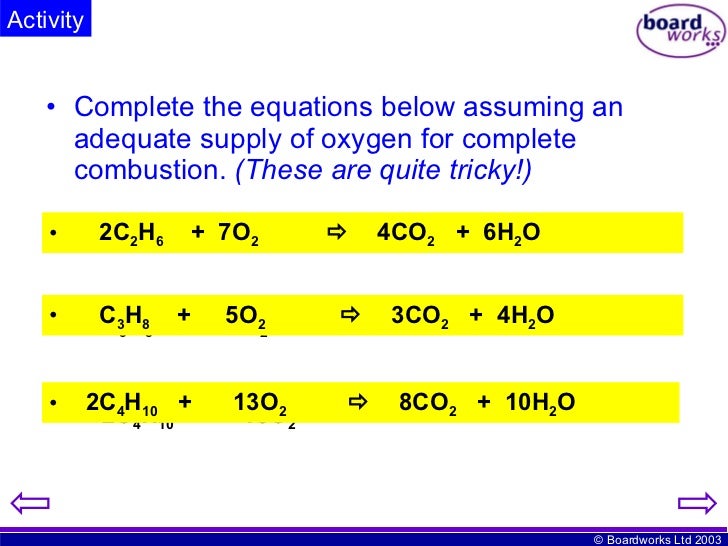
The term cracking is most commonly used to describe the breakdown of long-chain hydrocarbon fractions from crude oil into shorter-chain alkanes and alkenes. It is a type of thermal decomposition reaction. This also happens in cracking in chemistry.Ĭhemical cracking is the process of breaking down large molecules into smaller, more useful ones. In both cases, we break a larger object apart into smaller pieces to make it more useful. When we crack a coconut, we are rewarded with a feast of refreshingly cool coconut water. When we crack a Christmas cracker, we snap it in half to reveal a silly joke and paper hat. Finally, we'll learn how to balance cracking equations.We'll then explore two common types of cracking, including their processes.We'll define cracking before looking at its purpose and products.This article is about cracking in chemistry.Cracking an object into smaller pieces to make it more useful is much like chemical cracking. Sure, smashing a plate isn't much fun, but unless you break open an egg, you'll never be able to make an omelette with the tasty white and yolk inside.

The object smashes - it breaks into many tiny pieces.īut cracking doesn't have to be a bad thing. Reaction Quotient and Le Chatelier's PrincipleĬrack! Many of us have dropped something fragile on the floor before, be it a glass, a jar of jam, a plate, or an egg.Prediction of Element Properties Based on Periodic Trends.



 0 kommentar(er)
0 kommentar(er)
